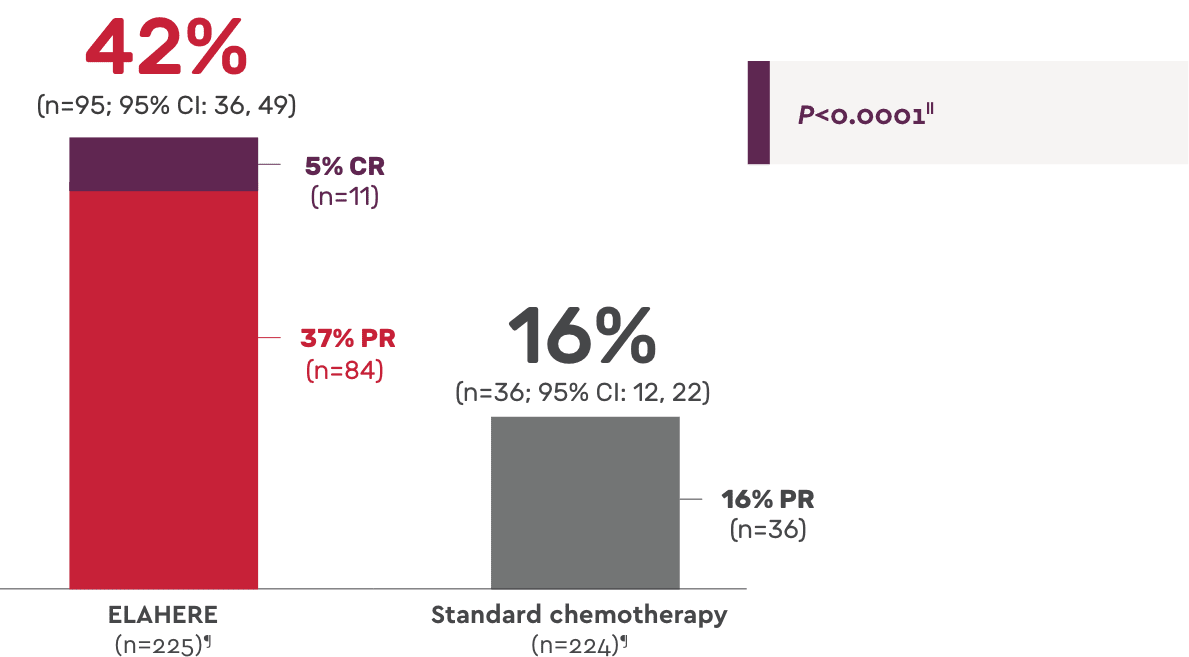THE FIRST TREATMENT TO SHOW STATISTICALLY SIGNIFICANT IMPROVEMENTS IN FRα+ PLATINUM-RESISTANT OVARIAN CANCER1-3
PFS: Statistically significant improvement vs standard chemotherapy1,2
ELAHERE reduced risk of disease progression or death by 35% vs standard chemotherapy1*
Primary endpoint: Progression-free survival with ELAHERE vs standard single-agent chemotherapy (ITT population)1
.png)
*Risk reduction derived from the
hazard ratio (HR=0.65).
†Two-sided P value based
on stratified log-rank test.
OS: First and only FRα-targeted treatment to deliver a statistically significant improvement in PROC2,3
ELAHERE delivered longer median overall survival than standard chemotherapy1
Secondary endpoint: Overall survival with ELAHERE vs standard chemotherapy1
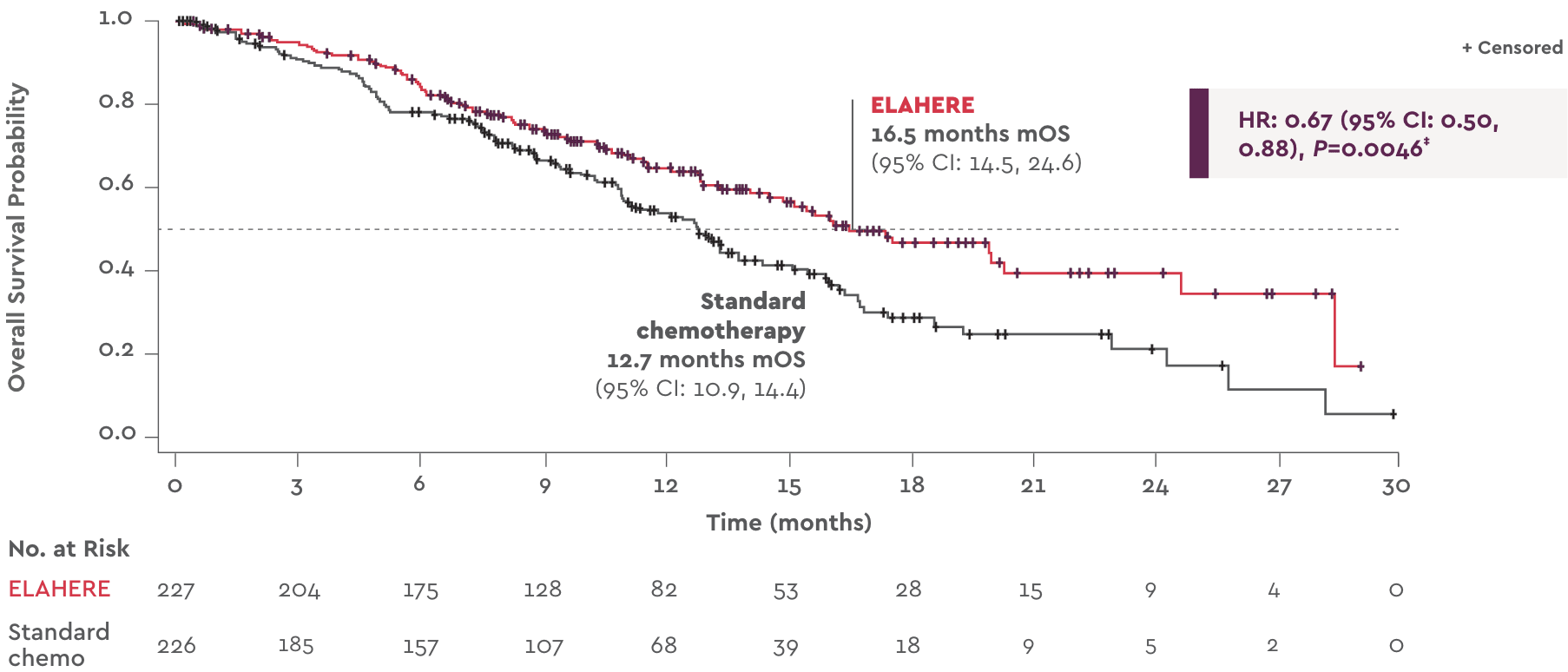
ELAHERE reduced risk of death by 33% vs standard chemotherapy1§
‡Two-sided P value
based on stratified log-rank test.
§Risk reduction derived from
the hazard ratio (HR=0.67).
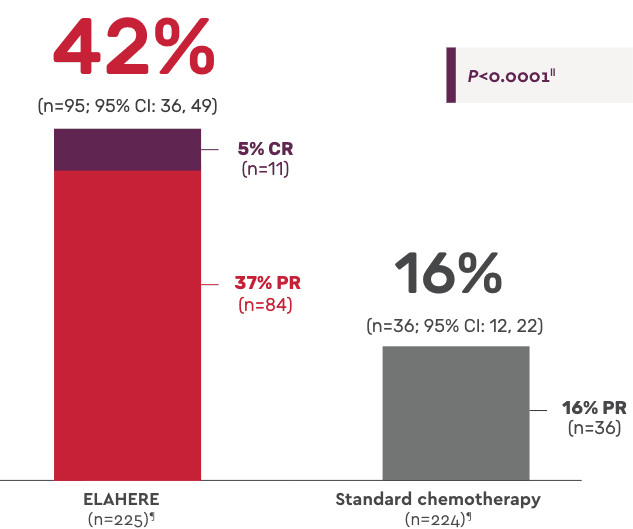
ORR: Statistically significant improvement vs standard chemotherapy1
Complete responses: 11 patients with ELAHERE vs 0 patients with standard chemotherapy4
Secondary endpoint: Overall response rate1,4
- Median duration of response: 6.77 months (n=95; 95% CI: 5.62, 7.89) with ELAHERE vs 4.47 months (n=36; 95% CI: 4.17, 5.82) with standard chemotherapy; HR: 0.64 (95% CI: 0.410, 0.993)4
- In the ITT population, 13.7% of patients treated with ELAHERE (n=31/227) had progressive disease vs 27.4% of patients treated with standard chemotherapy (n=62/226)2
- CA-125 response: 58.0% with ELAHERE (n=181; 95% CI: 50.5, 65.3) vs 30.3% with standard chemotherapy (n=155; 95% CI: 23.2, 38.2)2
‖Two-sided P value based upon Cochran-Mantel-Haenszel (CMH) test.
¶N values are based on the number of patients with measurable disease at baseline.
MIRASOL: The first and only positive phase 3 study vs single-agent chemotherapy in FRα+ PROC2
MIRASOL was a confirmatory, global, multicenter, randomized, open-label study evaluating the efficacy and safety of ELAHERE vs investigator’s choice chemotherapy in FRα-positive, platinum-resistant ovarian cancer2#
Patient population (N=453)
Key eligibility:
- Platinum-resistant disease: PFI ≤6 months
- FRα positive: ≥75% of viable tumor cells staining with ≥2+ intensity
ELAHERE (n=227)
(6 mg/kg AIBW q3w)
Investigator’s choice chemotherapy (n=226)**:
- 41% paclitaxel (n=92)
- 36% PLD (n=81)
- 23% topotecan (n=53)
Primary endpoint:
PFS by investigator
Key secondary endpoints:
- OS
- ORR by investigator
- PRO
- Patients received 1-3 lines of prior systemic therapy; prior bevacizumab and PARPi therapy allowed2
- Patients with BRCA mutations were allowed in the study2
- Patients with primary platinum-refractory disease, defined as progression-free interval <3 months, were excluded2
- Patients were excluded if they had corneal disorders, ocular conditions requiring ongoing treatment, Grade >1 peripheral neuropathy, or noninfectious interstitial lung disease1
ELAHERE received accelerated approval based on SORAYA, a single-arm study of patients with FRα-positive, platinum-resistant ovarian cancer (N=106). Patients had received up to 3 lines of prior systemic therapy and prior treatment with bevacizumab was required. The primary endpoint was investigator-assessed ORR and the key secondary endpoint was investigator-assessed DOR.1,3
#Includes epithelial ovarian,
fallopian tube, or primary peritoneal cancer.
**Paclitaxel was administered
intravenously on days 1, 8, 15, and 22 of a 4-week cycle (80
mg/m2 of BSA); PLD was administered
intravenously on day 1 of a 4-week cycle (40 mg/m2); and topotecan was administered intravenously on days 1, 8, and
15 of a 4-week cycle (4 mg/m2) or was
administered intravenously on days 1-5 of a 3-week cycle (1.25
mg/m2).2
MIRASOL patient characteristics2,5
| Characteristics, n (%) | ELAHERE (n=227) |
Standard chemotherapy (n=226) |
||||||||||||||||
| Median age (range) | Age in years | 64 (32-88) | 62 (29-87) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage at initial diagnosis |
|
|||||||||||||||||
| BRCA mutation |
|
|||||||||||||||||
| Prior exposure |
|
|||||||||||||||||
| Primary platinum-free interval |
|
|||||||||||||||||
| Platinum-free interval |
|
|||||||||||||||||
| Number of prior systemic therapies |
|
|||||||||||||||||
| Investigator’s choice of chemotherapya (Selected prior to randomization) |
|
|||||||||||||||||
| ECOG PS |
|
|||||||||||||||||
| Race |
|
|||||||||||||||||
| Ethnicity |
|
|||||||||||||||||
aInvestigators selected the chemotherapy prior to randomization in order to avoid selection bias.
NCCN Guidelines® V.3.2024 recommend mirvetuximab soravtansine-gynx (ELAHERE®) as a NCCN Category 1 preferred regimen for recurrence therapy in patients with folate receptor-alpha positive (FRα-expressing tumors [≥75% positive tumor cells]) platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer.6
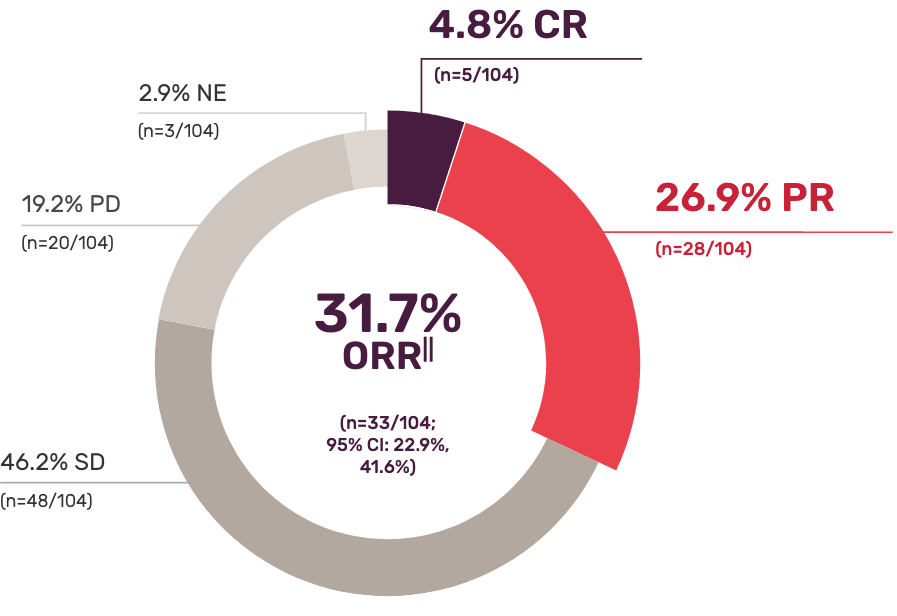
Single-arm study (SORAYA) results: ~1 in 3 patients achieved a complete or partial response with ELAHERE1††
(95% CI: 5.6, 9.7)
- Stable disease (SD) was an exploratory endpoint. It is unknown if SD is a result of natural disease progression or treatment with ELAHERE
- Consistent response rates and duration were demonstrated in the independent radiology review1
††Investigator-assessed per RECIST v1.1.
SORAYA: The first positive FRα biomarker-driven study1,2
SORAYA was a pivotal single-arm trial evaluating the efficacy and safety of ELAHERE in patients with FRα-positive, platinum-resistant ovarian cancer1,2
Patients with FRα-positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer (N=106)
ELAHERE 6 mg/kg AIBW‡‡ every 3 weeks, continued until disease progression or unacceptable toxicity
Primary endpoint3§§
- ORR (investigator-assessed)
Key secondary endpoint3§§
- DOR (investigator-assessed)
- Patients had received 1 to 3 lines of prior systemic therapy1
- Platinum resistance was defined as a disease recurrence within 6 months of treatment with platinum-based chemotherapy4
- Patients were excluded if they had corneal disorders, ocular conditions requiring ongoing treatment, Grade >1 peripheral neuropathy, or noninfectious interstitial lung disease1
‡‡Starting dose.
§§Investigator-assessed
per RECIST v1.1.
SORAYA patient selection and characteristics
ELAHERE was studied in a population with high unmet need3,4
- Patients with platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer who had 1 to 3 prior lines of therapy were selected for treatment with ELAHERE using the VENTANA FOLR1 IHC assay for FRα positivity1||||¶¶
| Baseline patient characteristics,(N=106)3 | ||||||||||||
| Age, median years (range) | 62 years (35-85) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Race, n (%) |
|
|||||||||||
| ECOG PS, n (%) |
|
|||||||||||
| BRCA mutation, n (%) | Yes | 21 (20) | ||||||||||
| Prior exposure, n (%) |
|
|||||||||||
| Prior systemic therapy, n (%)a |
|
|||||||||||
aOne patient had received 4 prior lines of therapy.3
||||Positive FRα expression of the tumor was defined as ≥75% of
viable tumor cells staining with ≥2+ intensity by the VENTANA
FOLR1 (FOLR1-2.1) RxDx Assay.1,3
¶¶The platinum-free intervals (PFIs) were 0-3 months in 37% (after
second- or third-line platinum-based chemotherapy) and 3-6 months
in 60% of patients, respectively.3
Discover the safety profile of ELAHERE
View Safety ProfileHow to Test for FRα
Testing for FRα is carried out at labs that have been validated to perform the VENTANA FOLR1 IHC assay.
How to Order ELAHERE
Please contact your participating specialty distributor or specialty pharmacy partners listed in the ELAHERE Ordering Information Sheet.
AIBW=adjusted ideal body weight; BRCA=breast cancer gene; BSA=body surface area; CA-125=cancer antigen 125; CI=confidence interval; CR=complete response; DOR=duration of response; ECOG PS=Eastern Cooperative Oncology Group performance status; FOLR1=folate receptor 1; FRα=folate receptor alpha; HR=hazard ratio; IHC=immunohistochemistry; ITT=intent-to-treat; mDOR=median duration of response; mOS=median overall survival; mPFS=median progression-free survival; NCCN=National Comprehensive Cancer Network® (NCCN®); NE=not evaluable; ORR=overall response rate; OS=overall survival; PARPi=poly(ADP-ribose) polymerase inhibitor; PD=progressive disease; PFI=platinum-free interval; PFS=progression-free survival; PLD=pegylated liposomal doxorubicin; PR=partial response; PRO=patient-reported outcome; PROC=platinum-resistant ovarian cancer; RECIST=Response Evaluation Criteria in Solid Tumors; q3w=every 3 weeks.